Fish Health
Many of the common fish health issues aquarist encounter can be prevented by providing the correct water chemistry, and a good aquarium set up for the species of fish they will be keeping. Fish that are kept in closed environments tend to pass diseases quickly do to small water volume and heavy fish populations. Parasites do not have to go far to find a host in an aquarium ecosystem. In the wild, large volumes of flowing water makes it difficult for parasites to reach epidemic proportions.
Aquarium fish are most often hatchery raised with some still collected from the wild. Fish are most often staged in a wholesale facility before they are shipped to other wholesalers throughout the world. Fish are often only kept at a wholesale facility for a few days before being shipped. While at wholesale facilities, fish are kept in high stocking densities that allow for the easy transmission of disease. Fish that are infected in these facilities are then sent to other wholesalers, thus infecting new fish. Most wholesale facilities do not quarantine new fish as this would be cost prohibitive.
Local wholesalers ship new fish the day after they have been received to your local fish stores. It is the local fish store that normally ends up treating newly arrived fish for diseases the fish picked up in transit. If you bring home fish from your local fish store that have been there for less than two weeks, your risk for bringing a disease home is greatly increased.
Typically the safest place acquire new fish is directly from a breeder. Buying breeder direct allows you to avoid the primary source of disease transmission, the wholesale chain.
The best way to prevent the spread of disease is to quarantine all new fish. A quarantine aquarium can be as small as 10 or 20 gallons, depending on the size of fish. It should be a cycled aquarium, with water chemistry similar to the water chemistry of the aquarium they are coming from. It is best if the quarantine aquarium has a tight lid on it to prevent any possibly contaminated water from escaping. The quarantine aquarium should not be near any display aquariums, and it is best if it is in another room or building.

Any nets, test kit vials, or other equipment should not be shared between the quarantine and display aquariums. When feeding fish, feed the quarantine tank last. When working in and around the quarantine tank make sure you wash your hands thoroughly when done.
Quarantine tank should not have any live plants in case the new fish will need treatment for parasites with medication that can harm plants. No carbon can be used in the system as it will remove medication from solution.
All new fish should be quarantined for at least three weeks, longer if the new fish is a species known to commonly carry a deadly virus. Adding praziquantel or other medication to the aquarium when new fish are added is acceptable to clean up any common parasites the fish may be carrying. Whatever medication you use it must be safe for the fish you are treating. Not all species of fish tolerate medication equally. Antibiotics should not be used unless a bacterial infection is suspected, and then for 10 days.
If necessary, you can slowly adjust the water chemistry in the quarantine tank to match the chemistry of the display tank they will eventually be going to. Adjusting water chemistry in the quarantine tank will prevent pH and osmotic shock when the fish are introduced to the display aquarium.
While a quarantine aquarium can prevent the spread of parasites and bacteria, it will often not expose any viruses the fish may be carrying if they are not showing any symptoms. The best way to check if the fish may be carrying a virus is to introduce a known naive (never exposed to the virus) fish. The naive fish should be added and kept in the quarantine tank for at least a week at the end of the quarantine period. If no symptoms develop within the week with the naive fish, the new fish should be safe to add to the display tank.
If fish are suspected of carrying a deadly virus, they should be destroyed. Fish that are carriers of a virus will continue to shed the virus as long as you have these fish, which will always put your healthy fish at risk as long as you keep them.
Most fish diseases an aquarium hobbyist will have to deal with will be with new fish that were brought home from their local aquarium store. Fish will normally show disease symptoms within the first two weeks from the date they were acquired.
Not all fish diseases are treatable, therefore it is always best to quarantine all fish before introducing them to a display aquarium. When treating with medication, the system cannot have any activated carbon, and if a ultraviolet sterilizer is used it must be turned off with most medications. Activated carbon will remove many types of medication from solution. Ultraviolet sterilizers will breakdown many types of medications, rendering them ineffective.
Many diseases can live at low levels within the aquarium ecosystem without becoming a life threatening infection until water quality deteriorates and becomes a stressor on the fish’s immune system. Many adult fish can tolerate low level of parasites, but that same low level can cause mass mortality in juveniles and fry.
New viruses are being discovered in ornamental fish on a regular basis since the mid 1990’s. Viruses have become common in many species of fish in the ornamental fish trade. They are often species specific, affecting only one species, but there are exceptions. Many viruses have high mortality rate, and any survivors then become carriers that can go on to infect naive fish. Ornamental fish viruses have been responsible for millions of dollars in hatchery loses. Many hobbyist have also experienced catastrophic losses to a virus.
Unfortunately viral diseases are not curable, and need to be isolated in a strict quarantine system. If you detect a virus in new fish in your quarantine system, all new fish should be euthanized with Tricaine methanesulfonate (MS-222) as soon as detected. Water that had fish in it with a deadly virus should be considered a biohazard to any fish of the same species if the disease is species specific.
Discus Plague Virus
A common discus (Symphysodon spp.) virus often referred to as discus plague has been in the hobby since at least the 1980’s if not longer. Discus plague is believed to be caused by a virus, as it has been found in many discus that have shown the classic symptoms (per conversation with Tom Waltzek, DVM, PhD, University of Florida). Discus that have contracted this virus have very obvious symptoms which include, dark background color, clamped fins, heavy body and fin mucus, and hiding in a dark corner of the aquarium. Discus plague has a high mortality rate, often 50 to 90%. Discus that survive the the initial symptoms, which can last up to three weeks, will become carriers of the virus.

The discus plague is highly contagious but appears to be only transmitted between discus. Unfortunately, any discus exposed to this virus should probably be destroyed to prevent the transmission to other fish. The disease still has a lot of study yet to be done on fish that have been infected, and how long they may shed the virus. It is currently unknown if the fish will always shed the virus or if the fishes natural antibodies will eventually eradicate the virus. It is known that discus will shed the discus plague virus for more than six months. Also unknown is, if a system has been exposed to the discus plague virus, and all fish have been removed, how long can the virus survive without a host.
You should never purchase a fish from a facility that has fish that exhibit symptoms of the discus plague virus in any of their tanks. The virus can be transmitted through water cross contamination, so any water in a facility that contains fish with the discus plague virus can potentially introduce the virus to your systems even without the fish.
There is currently no cure for the discus plague virus. The virus might respond to heat therapy, by increasing the temperature to 90º F (32º C) as there are other viruses in the same family that have responded to heat. The tank must have good water circulation when using heat therapy as water will have a lower oxygen carrying capacity. Having antibiotics on hand to treat any secondary infections is a good idea, as bacterial infections often occur in discus when a heavy slim coat appears.
Angelfish Virus
Angelfish also have a related virus that causes the same symptoms as the discus plague virus. The angelfish virus should be treated in the same way as the discus plague virus.
Koi Herpesvirus

Koi herpesvirus (KHV, cyprinid herpesvirus-3 or CyHV3) was first discovered in England in 1996. KHV is highly contagious, commonly causing mortality in greater than 95% of koi and common carp (Cyprinus carpio) it infects. Infected koi are reported to die within 24 to 48 hours after first symptoms are observed. Most obvious symptoms are swimming lethargically and staying near the surface. The easiest way to detect a possible KHV infection is to check the gills for dead white patches (normal is red), and in some cases sunken eyes.
KHV infection typically does not show symptoms below 72° F (22° C) or above 86° F (30° C). It takes up to two weeks for koi to show symptoms of KHV in temperatures between 72° and 78°F (22° and 25.5°C). Once koi are infected with KHV it is believed that they will become carriers of the virus for life. In California, some koi hatcheries have been shutdown by the State, and made to destroy all fish, resulting in severe economic hardship for farmers. KHV has been known to still be active in a system for up to three days after the fish have been removed.
Lab tests have been developed to verify KHV infection. A KHV vaccine has been developed that has shown positive results in adult koi, but has caused mortality in some juveniles.
Goldfish Herpesvirus
Goldfish herpesvirus (GHV, cyprinid herpesvirus-2 or CyHV-2) causes the same symptoms as KHV, but only in goldfish.
Dwarf Gourami Iridovirus
Dwarf gourami iridovirus (DGIV) was believed to infect only the dwarf gourami (Colisa lalia, also known as Trichogaster lalius), but later studies have shown that it may be the same virus (or very closely related virus) that infects many other species, both freshwater and saltwater. It is believed that the virus originated in with imported dwarf gouramis from Singapore. The iridovirus is spread through the water. Other fish that are believed to be susceptible to the virus are swordtails (Xiphophorus hellerii), mollies (Poecilia spp.), guppies (Poecilia reticulata), cleaner wrasse (Labroides dimidatus), and banggai cardinalfish (Pterapogon kauderni).

DGIV has often been misdiagnosed as a bacterial infection. DGIV causes lesions on the fish that look similar to fish TB (Mycobacterium marinum). It causes 90% or more mortality in fish exposed to the iridovirus.
Ich (Ichthyophthirius multifiliis)
Ich (Ichthyophthirius multifiliis) is the most common parasite hobbyist will have to face. Ich is a microscopic parasite that you will see evidence of on the fish when it is near the end of its lifecycle. Small white dots will appear on the fish when ich is present. The ich parasite not only attacks the fins and body of the fish, but it also the gills. When the ich reaches significant numbers, it will make it difficult for the fish to respire. If not treated early, the parasite can kill all the fish in the aquarium. When observing fish in the aquarium store, be aware that not all infected fish will show spots on the body or fins, they can also be in the gills where it can go undetected.
Ich has a lifecycle that has several stages. The stage that is visible to the hobbyist is when the parasite has matured on the fish and forms a trophont. The trophont is buried under the fish’s skin and mucus and is protected from medication that is in the water. After ich reaches maturity on the fish, it will drop off and sticks to a surface area in the aquarium. It will then break open and hundreds of tomites will then swim through the water column seeking a fish to parasitize. It is the free swimming stage that is killed by medication.
There are several medications to treat an ich outbreak, as well a heat that have proven effective. All treatments need to be used with some caution, as not all medications are safe for all fish species.

Increase the water temperature to at least 85°F (30°C) when treating for ich as this will speed up the lifecycle and shorten the amount of time it will take to eradicate the parasite. Most tropical fish can tolerate 85°F (30°C) without any issues. Some people have had success in treating ich with 90°F (32°C) temperature only for about two weeks, but it is not guaranteed. Most tropical fish can tolerate 90°F (32°C), but there may be some that can be distressed by that high of temperature. If fish show signs of being distressed by high temperature, you should take immediate action to lower it through a cold water change. Increasing the water temperature will lower the oxygen carrying capacity, so make sure you have strong water flow in the aquarium during treatment.
Salt (sodium chloride, NaCl) is the safest medication to use with many fish. Fish that come from natural waters that are hard and alkaline are usually very salt tolerant. Prime candidates for salt treatment are cichlids from Lake Malawi, Lake Tanganyika, Lake Victoria, Central America, and livebearers such as guppies, platies, swordtails, variatus, and mollies. The "specific gravity" measured with a standard marine aquarium hydrometer should be between 1.005 and 1.009 or 7 to 13 ppt. To achieve this level of salt you will need to add 1 to 2 tablespoons per gallon of water. This level of salt is also known to kill many other freshwater fish diseases. Fish should be kept at this salt level for three weeks at 85°F (30°C). Salt is not safe for aquarium plants. Salt does not evaporate, and will need to be removed by water changes once treatment has been completed.
Copper will kill ich. Copper comes in many forms, and is produced as a medication for both freshwater and marine aquarium fish. Many fish are copper sensitive, and will show signs of stress within a couple of hours after of the medication has been added to the aquarium. The safest form of copper in my experience is “Copper Power.” I have been able to use Copper Power “Marine” version successfully with all freshwater fish, even with fish that had very bad reaction to other forms of copper medication.
Using copper as a medication requires the use of a copper test kit to measure the content of copper in solution in the aquarium water before adding more. Aquarium Pharmaceuticals (API) makes a copper test kit that is easy to use and is available at many aquarium stores.
Freshwater fish that I know from experience that can be killed from some forms of copper medication are tiger barbs (Puntius tetrazona), tinfoil barbs (Barbonymus schwanenfeldii), bala sharks (Balantiocheilos melanopterus), chinese algae eaters (Gyrinocheilus aymonieri), white cloud mountain minnow (Tanichthys albonubes), clown loaches (Chromobotia macracanthus), red fin blue botias (Yasuhikotakia modesta), and zebra danio (Danio rerio).
Fish should not be exposed to copper for longer than four weeks. Long term exposure to copper can cause neurological problems in fish, but it can be reversed if the fish are returned to a near copper free system.
Malachite green is also used to kill ich. Malachite green requires regular daily doses to maintain an effective level. Some fish are sensitive to malachite green, which includes many catfish species. Malachite green is often combined with formalin (formaldehyde), as it is believe the combination of both is more effective.
Velvet Disease (Piscinoodinium pillulare, Oodinium)
The velvet parasite has a life cycle similar to ich. When fish are heavily infected with the velvet parasite they take on a white velvety appearance. Individual velvet parasites are difficult to see as they are much smaller than ich parasites. It often goes undetected until numbers on the fish give it velvety appearance. The velvet parasite can live on the skin, gills, and intestines of the fish. The velvet parasite is photosynthetic, and derives some of its nutrition from light.
When treating for velvet disease, it is recommended that the aquarium lights be turned off so the parasite cannot photosynthesize.
Heat therapy can kill velvet disease provided the fish you are treating are able to tolerate the higher temperature. The temperature of the water should be increased to 91° to 93°F (33° to 34°C) and maintained for 48 hours. Fish that are good candidates for heat therapy are discus, rams, and angelfish.
Salt (sodium chloride, NaCl) is effective provided the fish you are treating can tolerate a specific gravity measured between 1.005 and 1.009 or 7 to 13 ppt. To achieve this level of salt you will need to add 1 to 2 tablespoons per gallon of water. Salt treatment should be maintained for three weeks. Once treatment is complete salt will need to be removed through water changes.
Copper can also be used to kill velvet disease. See copper treatment for ich for recommendation on how to treat with copper.
Other medications that can be considered are acriflavine, malachite green, and formalin.
Chilodonella (Chilodonella cyprini)
Chilodonella is a protozoan that causes a cloudy mucus looking patch on the fish’s body, or cloudiness in the fins. It can attack the skin, fins, and gills of the fish. Chilodonella can move to other fish within the system. Chilodonella is typically only an issue when the aquarium water is in poor condition. Overcrowding can increase the chance that Chilodonella can become a problem.
Salt (sodium chloride, NaCl) is effective provided the fish you are treating can tolerate a specific gravity measured between 1.005 and 1.009 or 7 to 13 ppt. To achieve this level of salt you will need to add 1 to 2 tablespoons per gallon of water. Although salt treatment has proven 100% effective in 24 hours in grass carp (Ctenopharyngodon idella) it is recommend it maintained for three weeks to treat Chilodonella. Once treatment is complete salt will need to be removed through water changes.
Malachite green, and formalin have also been effective against Chilodonella.
Skin Flukes (Gyrodactylus) and Gill Flukes (Dactylogyrus)
Skin flukes and gill flukes can live on aquarium fish at low enough levels that the fish do not display any symptoms of the parasite being present. Under poor water conditions or heavy population loads, skin flukes and gill flukes can reach very high numbers and cause severe distress or even mortality for fish. Typically, the symptoms that can be expressed are rubbing on rocks, wood, or substrate, rapid breathing, loss of appetite, weight loss, discolored gill lamellae and filaments, breathing through only one side (gill operculum [cover] clamped shut). Skin and fins may look cloudy or not as bright as normal. Fins on some fish may be clamped.
Both skin and gill flukes have a rostral hooked organ that attaches to the fish. The hooks puncture the skin or gills causing damage and irritation. The damaged tissue from skin and gill flukes can cause secondary infections and inhibit the ability of gills to extract oxygen from the water.
While both gill and skin flukes look similar under the microscope, there are differences. Skin flukes are live bearing and gill flukes are egg layers. Skin flukes have four eye spots near the non grasping end of the parasite. Because gill flukes are egg layers, multiple treatments are often necessary in order to eradicate the parasite. Medication often do not kill the eggs of gill flukes.
Discus and other cichlid breeders often have problems raising fry because of gill flukes in their breeding systems that attack newly hatched fry. Flukes can live on the parent fish and be passed to the fry. Adult fish often do not express any symptoms of infection, but can pass the flukes on to newly hatched fry. Because fry are so small, it does not take many flukes to cause mortality. Slow growth rate of juvenile fish can also be caused by flukes.
The preferred and safest treatment for flukes is praziquantel. Praziquantel is commonly available in the aquarium hobby as PraziPro. PraziPro is extremely safe and effective. The only side effect observed when treating with praziquantel was a temporary cessation of fish reproduction for about two months post treatment in livebearers.
Salt (sodium chloride, NaCl) is effective provided the fish you are treating can tolerate a specific gravity measured between 1.005 and 1.009 or 7 to 13 ppt. To achieve this level of salt you will need to add 1 to 2 tablespoons per gallon of water. Fish should be kept at this salt level for three weeks at 85°F (30°C). Once treatment is complete salt will need to be removed through water changes.
Organophosphates like trichlorfon can be used to kill gill and skin flukes.
Formalin can be used to treat flukes. If treating gill flukes, it will typically require three days of treatment followed by a three day break, then three more days of treatment to kill any that have hatched from eggs. Some fish are sensitive to formalin.
Baths in medicated water are not an effective way to eliminate flukes. The aquarium system must be treated to eliminate gill or skin flukes that may not be attached to fish.
Tapeworms (Diphyllobothrium sp.)

Tapeworms are a white segmented (band shaped) worm that live in the digestive tract of fish. They can rob fish of nourishment from the food they eat, and make the fish’s abdomen look full when it is really full of tapeworms. Typically the hobbyist will not know the fish has tapeworms until the fish begin to pass some segments. Tapeworms can be several inches long, flat and segmented.
California blackworms (Lumbriculus variegatus) are a common source of introducing tapeworms to the aquarium system. Discus are the most common fish in the hobby to be a host to tapeworms.
Luckily, tapeworms are easily treated with praziquantel (PraziPro). Tapeworm are very sensitive to praziquantel, and it is not unusual for fish to start passing tapeworms within 30 minutes of being exposed to the medication. Discus hobbyist can be surprised by how many of their fish are carrying tapeworms when the aquarium is treated with praziquantel.
Capillaria
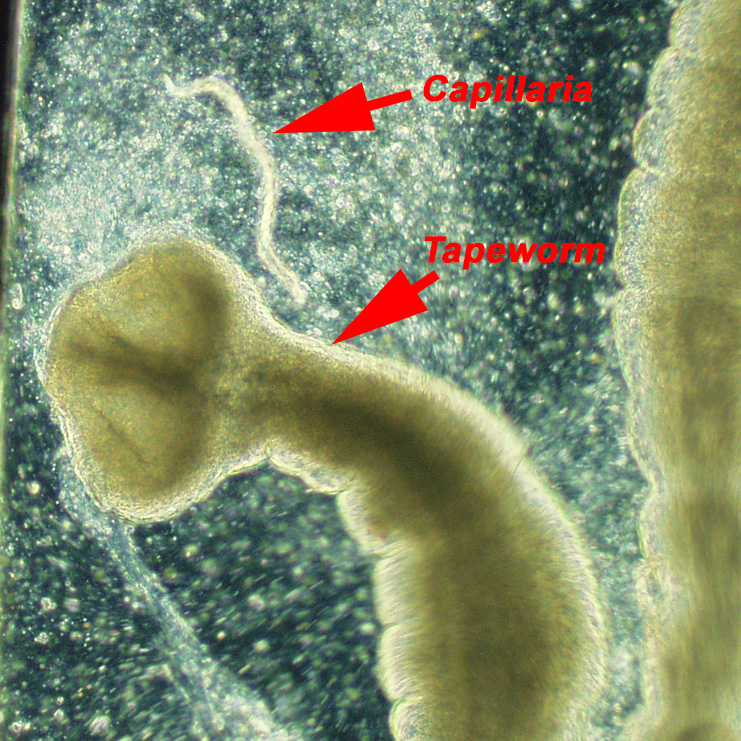
Capillaria are a common parasite that is found in the digestive track of many species of fish. The worms can be transmitted to fish by feeding live tubifex and California blackworms. Fish can also contract the parasite by ingesting Capillaria eggs excreted by infected fish housed in the same aquarium.
Symptoms of a severe Capillaria infection include, fish turning dark, becoming emaciated, and lethargic. Discus as well as other tropical fish that have a heavy population of Capillaria in the gut will often pass white stringy feces.
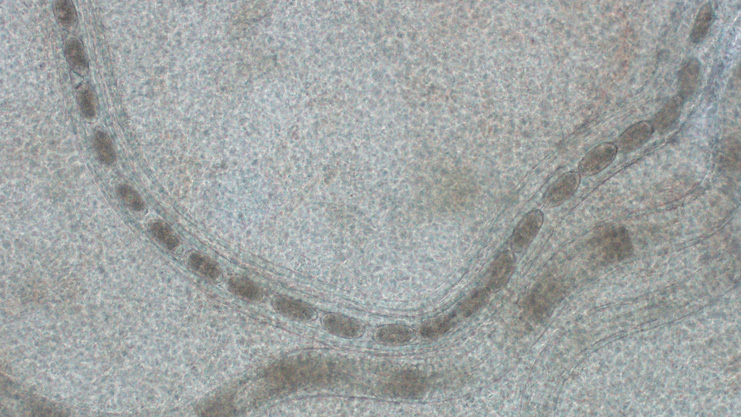
Diagnosis of a Capillaria infection can be done by examining gut contents of a dissected fish under a microscope and finding the worms or it's eggs. Fecal discharge can often contain the eggs of Capillaria. Capillaria eggs look like medicine capsules under a microscope.
Treatment for Capillaria includes heat therapy 90°F (32°C) and/or medicating with praziquantel (PraziPro). Other medications that may be effective include fenbendazole, trichlorofon, and mebendazole.
Spironucleus

Spironucleus is often mis-identified as hexamita in fish. Both hexamita and spironucleus look very similar under the microscope, but recent research suggests that only spironucleus is found in tropical fish. Spironucleus is an internal parasite that is often found in seemingly healthy fish. Spironucleus is commonly seen in discus, but it is also in other fish. When environmental condition deteriorate spironucleus can infect many organs of the fish. The most common symptoms of a severe infection of spironucleus are white translucent feces, fish stop eating, and the fish becomes emaciated. Hole in the head (HITH) symptom (an environmental condition) is often incorrectly attributed to spironucleus.
If one fish in the aquarium shows symptoms of spironucleus there is a good chance that many if not all are carrying the parasite. It is recommended that the nitrate level be checked and lowered through water changes if necessary to 5 ppm or less. Heat therapy is the first choice for treating spironucleus. Spironucleus can be treated with 90°F (32°C) for three weeks. Discus that are not eating will often respond to heat therapy within the first week.
Metronidazole is the medication of choice for treating spironucleus. It is most effective if it is consumed with food. To prepare medicated food, dissolve 250 mg of metronidazole in a cup of warm water. Food can be soaked in a solution containing metronidazole for five minutes before it is fed to fish. Fish should be fed three times a day with metronidazole laced food.
If fish are not eating, add 250 to 500 mg of metronidazole per 10 gallons (4 l) of water. Medication should be added every day. It is believed that metronidazole can be adsorbed through the gills of the fish. Aquarium water should be kept at >82°F (28°C) as some have suggested that it may precipitate out of solution at lower temperature. Treatment with metronidazole should be continued for five to 10 days. Metronidazole is light sensitive, therefore for the most effective treatment in the aquarium it is best to turn the lights off while medicating the water.
A combination of heat therapy and metronidazole can be used if desired.
Neon Tetra Disease (NTD, Pleistophora hyphessobryconis)
Neon Tetra Disease (NTD) is caused by the Pleistophora hyphessobryconis protozoan. It is called neon tetra disease because it was first identified and is commonly found in neons (Paracheirodon innesi). NTD can infect many species of fish. NTD parasite causes an opaque white discoloration in the muscle tissue of the fish. It can live within the fish at a low level and go undetected until large amounts of tissue are dead.
NTD is spread by fish ingesting the protozoan with food or eating fish (live or dead) that are infected with the parasite. Fish that are infected with the parasite need to removed from the system immediately, and euthanized as NTD has no cure.
Fungus is a secondary infection that looks like a white cottony patch. Fungus will grow on dead tissue often the result of trauma caused by other fish, or a bacterial infection. Fungal infections are not contagious.
Before treating for fungus you must first determine the cause. It is best to move the infected fish to a quarantine tank for treatment. Typically fungus grows on dead tissue that has a bacterial infection. Fish that have a fungal infection should be treated with an antibiotic such as penicillin or ampicillin, along with methylene blue.
Columnaris (aka. Flavobacterium columnare, Flexibacteria, Fin Rot, Tail Rot, Mouth Rot)

Columnaris causes a white patch on the fish often resembling a saddle. Columnaris is a more solid white in the middle and fades outward. Columnaris is a gram negative bacteria, and when viewed under a microscope looks like rods. Fish that are exposed to high ammonia levels or low oxygen levels are at greatest risk of contracting columnaris. It is most commonly seen on livebearers that have been newly acquired. Columnaris will normally appear on fish within the first week of acquisition. It needs to be treated as soon as it is noticed. Mortality can occur in fish that contract columnaris within three days. Columnaris can quickly spread in warm water. Columnaris is a freshwater fish disease that cannot tolerate salt.
Columnaris can be treated with oxytetracycline, neomycin, kanamycin, and salt (sodium chloride). Hydrogen peroxide can be applied to areas of infection with a cotton swab to kill bacteria. Hydrogen peroxide can be purchased off the shelf at your local pharmacy or grocery store.
If possible lower the water temperature to 75°F (24°C) to slow the reproduction rate of the columnaris bacteria. Salt should be the first choice for treating columnaris provided the fish you are treating are salt tolerant. Salt concentration should be at least 5 parts per thousand (ppt) but preferably 10 ppt would be best. This equates to about 1 to 2 tablespoons of salt per gallon. You should use a marine aquarium hydrometer to check the salt concentration of the water. Fish should be treated for at least 10 days, then the water can be returned to normal freshwater through water changes. Fish should be closely observed for three weeks post treatment to make sure there is no recurrence of columnaris.
Some symptoms and conditions fish can display can be directly attributed to water chemistry. Often symptoms expressed by fish are misdiagnosed as being caused by a disease.
Shimmies
Shimmies describes abnormal swimming behavior of many species of livebearers, including guppies (Poecilia reticulata), mollies (Poecilia sphenops, Poecilia velifera, Poecilia latipinna), swordtails (Xiphophorus hellerii), platies (Xiphophorus maculatus), and variatus (Xiphophorus variatus).
The predominant cause of shimmies in livebearers is a water chemistry that is too soft and acidic. Mollies are the most common fish to exhibit the shimmies symptom as they like very hard and alkaline water. Increase water hardness as outlined in this book to 300 to 600 ppm (17° to 34° dGH) and increase alkalinity (pH) to 90 to 160 ppm (5° dKH to 9° dKH). pH will be affected negatively by any nitrate in the water. The higher the nitrate concentration the lower the pH. Maintaining a pH of 7.6 to 8.3 is recommended for most livebearers and for mollies a minimum of 7.8 is recommended.
Hole in the Head (HITH), Chronic Ulcerative Dermatopathy (CUD), Chronic Erosive Dermatopathy

Hole in the head describes a symptom (not a disease) that can develop in fish in the aquarium due to poor water quality. Hole in the head only occurs in aquarium kept fish, it is not found in wild fish populations. Marine hobbyist have a similar condition to hole in the head, but it is commonly referred to as head and lateral line erosion (HLLE). The symptom looks like pits in the head or receding skin on the head and sometimes the lateral line. In freshwater the Hole in the head symptom is most commonly seen in large cichilds, including oscars (Astronotus ocellatus), and discus (Symphysodon spp.).
Hole in the head is caused by excessive nitrate in the aquarium system. In aquariums where the hole in the head symptom develops it is not unusual to have over 400 ppm nitrate in the water. When fish feed in systems with high nitrate water they take in small amounts of water containing nitrate. Anaerobic bacteria in the digestive tract break down the small amount of nitrate into nitrite. Nitrite is adsorbed by the the red blood cells of the fish, creating a small amount of Methemoglobin. The small amount of Methemoglobin is not enough to kill the fish, but causes a chronic low oxygen level in the circulatory system. The fish's tissue uses the oxygen as the blood circulates, but by the time it gets to the outer extremities of the head and the lateral line, only Methemoglobin remains in the blood. Tissue around the head and/or lateral line will start to die do to the lack of oxygen in the blood.
Hole in the head can sometimes open the fish up to secondary infections that are often incorrectly blamed for the symptom.
Hole in the head can be reversed by conducting large percentage water changes. Greater than 90% water changes may need to be done to bring nitrate down to an acceptable level. Caution must be used when lowering excessive nitrate levels through water changes as nitrate lowers pH. When large water changes are done the pH will be higher after the water change due to the reduction of nitrate. Small 25% water changes may need to be done daily over the course of a week or two to slowly bring the pH up, before large water changes can be safely done to avoid pH shock. Adding sodium bicarbonate to the aquarium over several days to stabilize pH before large water changes are done, is another technique that can be used to avoid pH shock.
Large cichlids produce a lot of waste that will eventually turn into nitrate when the aquarium ecosystem is out of balance. Nitrate must be controlled with large frequent water changes and not allowed to exceed 100 ppm for longer than a couple of weeks. Maintaining low nitrate levels, below 40 ppm, is all that needs to be done to reverse the tissue damage. Secondary infections may require an appropriate medication.
Hole in the head and head and lateral line erosion are perhaps the most incorrectly attributed symptom to some other cause. The list of causes include spironucleus/hexamita, carbon filtration, stray voltage, and nutritional deficiency.
Since spironucleus occurs in wild fish, and wild fish never show the HITH/HLLE symptom it cannot be the cause. Many samples have been taken from the holes in aquarium fish that have often come up negative for disease. Many fish that have tested positive for spironucleus have no HITH pits.
Carbon has been suggested as a possible cause of HITH/HLLE, but it cannot be the cause since it occurs also in fish that are in systems that have no carbon filtration.
Stray voltage cannot be the cause of HITH/HLLE because reversal of the condition can take place in the same system where it developed without changing out any equipment.
Nutritional deficiency has often been suggested as a possible cause of HITH/HLLE, but fish that do have excellent diets can also show the symptom. Nutrition should always be balanced for the fish you are keeping. Even fish that are carnivores get vitamins from fish they eat that many have vegetable matter as large source of their diet. Some fish that aquarist think of as being strictly as carnivores, like many Central American cichlids will eat vegetable matter like nori seaweed, and boiled spinach as part of their regular diet.
Osmotic and pH Shock
Osmotic and pH shock occur in fish when the water chemistry drastically changes in a short time span, typically due to a water change or move to another aquarium. Symptoms are usually expressed by fish becoming lethargic, laying on the bottom of the tank, and heavy respiration. Death can occur within 24 hours if the fish are not returned to the original water chemistry they have become accustomed to.
Water chemistry should be adjusted slowly over a week or two when a major change is desirable. pH shock is common when a hobbyist does not do regular scheduled water changes and then tries to make up for poor maintenance practice with one large water change. This typically causes a drastic change in pH due to the new water not containing the pH reducing nitrate.
Bringing fish home from the aquarium shop is also a common factor in osmotic and pH shock. Not all aquarium/pet shops pay attention to the water chemistry of their aquariums. If the fish you are buying are kept in poor or incorrect water conditions, and you add them to correct/good water conditions they can experience osmotic and pH shock. It is a good idea to test the water in the bag for pH, and hardness before adding them to your aquarium. Adjust your aquarium water chemistry to be within 100 ppm hardness and .5 pH to avoid osmotic and pH shock.