Nitrogen Cycle
When starting a new aquarium there are some very important things you need to know before adding fish to the aquarium. Understanding the nitrogen cycle and how it works is fundamental if you plan on being a successful aquarium hobbyist. Having a large filter on your aquarium in itself will not keep your fish alive. Aerobic beneficial bacteria are required to keep your aquarium hospitable for fish. These beneficial bacteria live on all surface area within the aquarium including the parts of the filtration system that is in contact with oxygen rich water. These aerobic bacteria are what is often referred to a biological filtration.
When starting a new aquarium, the tank is nearly sterile. During the first 35 days after the aquarium was set up (with fish) is the most difficult time for fish to survive. During this time bacteria population try to catch up with the fish population load. There are three chemicals that make up the nitrogen cycle the hobbyist needs to monitor, ammonia, nitrite, and nitrate. Ammonia, nitrite, and nitrate concentrations can be monitored with aquarium test kits. Every aquarist should have a aquarium test kit available that can test for ammonia, nitrite, nitrate, and pH. Master test kits are available from many companies that include tests for ammonia, nitrite, nitrate, and pH.
Fish excrete ammonia through respiration and waste. Ammonia at high enough concentrations can damage the fish's gills and other organs, making it difficult to take in oxygen. Bacteria known as Nitrosospira and Nitrosomonas help oxidize ammonia into nitrite in the aquarium. When you test for ammonia with your aquarium test kit, the reading you actually have is a combination of ammonium (NH4+ or ionized ammonia) and ammonia (NH3 or unionized ammonia) known as Total Ammonia Nitrogen (TAN). Ammonia is the toxic part of the TAN.
Ammonium even at highest concentrations you would see in the aquarium does not cause mortality in fish. Understanding the difference between the two is crucial to figuring out how much toxic ammonia you really have in your system. Ammonia concentration of as little as .6 ppm is toxic to many species of fish.
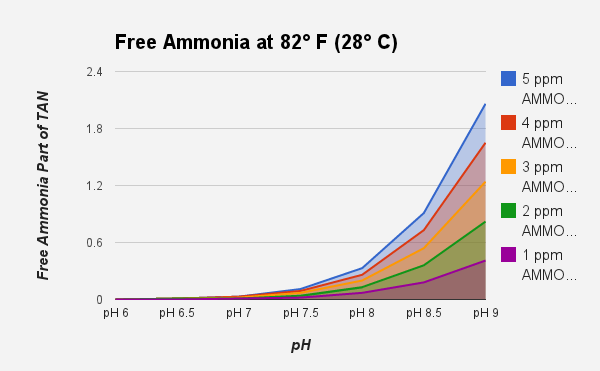
If you are trying to keep Tanganyikan cichlids in water with a pH of 9.0, that has a TAN of 5 ppm your ammonia level is 2.06 ppm (a deadly danger zone). This is why saltwater fish and African cichlids are thought to be more sensitive to ammonia, these fish are normally maintained in water with a pH of 8.2 or greater. At a pH of 6.0, and 10 ppm of TAN, the ammonia is only .007 ppm. While it looks like the fish mortality should be very high, the fish are doing fine. The graph below provides a "True Free Ammonia" chart that can be referenced for figuring out how dangerous your TAN reading is.
South American and West African cichlid breeders that maintain a low pH below 6.0 need to be cautious when performing water changes, as the low pH has an adverse affect on the nitrifying bacteria that converts ammonia to nitrite. Because of the acidity, these bacteria populations can drop so low that the TAN reading can rise quickly. While the pH stays low the TAN reading is nearly all ammonium, but if you do a water change or add a alkalinity buffer to the system the ammonium can be quickly converted to ammonia, potentially causing ammonia poisoning.
Free Ammonia Part of TAN Calculator
If you know your temperature in Celsius skip "Temperature Conversion" fields. Enter your ammonia test result in the first field, then enter the pH from test result in the second field, then enter the temperature in °C in the third field. Click on "Calculate" button to see the amount of toxic ammonia in the TAN. A NH3 level of .6 or greater can kill many species of fish within 24 hours of exposure.
Ammonia levels will normally peak around 2 weeks after adding the first fish. In an established aquarium the ammonia level should always read 0 ppm. Often in freshwater aquariums, fish will survive the ammonia spike when a new tank is cycling, because the pH is low enough that the concentration of true ammonia stays low. It is the nitrite that is more often the killer of fish in a new aquarium setup.
In an established aquarium nitrite level should always be at 0 ppm. Nitrospira bacteria oxidize nitrite into nitrate. Nitrite is extremely toxic to fish. It only takes .5 ppm of nitrite to cause stress, and 2 ppm to kill fish. Nitrite in the water will displace oxygen in the fish's blood, causing a condition called methemoglobinemia or “brown blood disease.” Nitrite is primarily absorbed through the gills and digestive tract and attaches to hemoglobin. This prevents the uptake of oxygen.
Nitrite levels will peak around three weeks after adding fish to a new aquarium setup. It is extremely important to note that nitrite is the number one cause of fish mortality in a new freshwater aquarium setup (New Tank Syndrome), not ammonia.
Nitrate is final chemical in the nitrogen cycle. In most freshwater aquariums, nitrate builds up over time. The rate of accumulation of nitrate is directly related to fish waste, decay of aquatic plant matter, and if there are any natural nitrate reduction (NNR) strategies employed within the aquarium ecosystem. Aquariums with a heavy fish load, that are fed often with high protein food, can accumulate nitrate very quickly, often well over 100 ppm in a month.
In natural bodies of water, nitrate is rarely detectable (<1 ppm) with an aquarium test kit.
While nitrate is not as toxic as ammonia and nitrite it can have an adverse affect on the aquarium ecosystem. Fish that are exposed to very high nitrate levels (> 400 ppm) for extended period of time have been know to develop a hole in the head (HITH) condition where the skin around the head recedes. HITH condition is commonly seen in Oscars (Astronotus ocellatus), and other large Central and South American cichlids. Excessive nitrate can affect reproduction, fish growth rate, and the immune system's ability to protect against disease.
Keeping nitrate as close to 0 ppm is optimal. As nitrate increases it will also lower the aquarium's pH. This can be a problem for fish that are adapted to a higher pH range and in systems that have low alkalinity. In freshwater aquariums, nitrate should not be allowed to exceed 100 ppm for more than a month. In systems that have a low alkalinity level, 100 ppm of nitrate can easily lower the pH below 6.0. Low pH has been linked to the lower oxygen carrying capacity of the fish’s blood by as much as 60%. A low pH and high nitrate level is double trouble for the fish’s ability to take up oxygen.
Nitrate typically builds up in the aquarium over time, but it can be slowed down and even completely kept from rising. NNR can be accomplished by implementing nitrate reducing elements into your aquarium ecosystem, and keeping fish populations (biomass) in check.

Fast growing live plantsunder bright light will take up nutrients from the decomposition of fish waste, uneaten food, and plant matter. The brighter the light, the faster the plants will take up nitrate. An aquarium positioned in the home so that it will receive a couple of hours of natural sunlight can help reduce nitrate, as well as help bring out the natural colors of fish. Most species of aquatic plants prefer ammonium over nitrate. As fish release ammonia, most of it will be converted to ammonium and thus available for plants as a nutrient. Plants in a brightly lit aquarium are one of the best ways to control nitrate accumulation in the freshwater system.
Hydroponics system can also be added to an aquarium ecosystem to control and reduce nitrates. Hydroponics system method may be the best for aquarium systems that have a high bioload like tanks with large cichlids, and growout tanks.
A substrate comprised of fine sand can also help with denitrification. Anaerobic bacteria live in areas devoid of oxygen. Anaerobic bacteria use the oxygen attached to the nitrate freeing up nitrogen gas that then can escape into the atmosphere. Anaerobic bacteria will colonize the depths of a sand bed. Using Malaysian live bearing snails (Melanoides tuberculata) to slowly turn over the sand bed will help exchange nitrate rich water through the sand to the anaerobic bacteria. Catfish from the Corydoras genus as well as other small sand sifting fish can also help get nitrate to the anaerobic bacteria.
It is recommended that you have at least a 1 ½ to 2 inches (3 to 5 cm) of fine sand. Gravel allows the exchange of oxygenated water to cycle to easily through the substrate for it to be useful for NNR without requiring a significant depth. The finer the substrate the shallower the depth needs to be to achieve some denitrification. The pH also affects anaerobic bacteria population. To maintain a healthy anaerobic bacteria population the pH should always be kept on the alkaline side, as they do like acidic water.
When fish start to die in an aquarium that has been set up for less than a month, it is often referred to as “New Tank Syndrome.” Toxic levels of either ammonia or nitrite are what causes new tank syndrome in an aquarium that does not have an established nitrogen cycle. Typically, if fish show stress or start to die within two weeks or less, it is a toxic level of ammonia. At two to four weeks after the aquarium is set up with fish, it is typically nitrite poisoning causing stress and mortality.
When you start a new aquarium, it takes an average of 35 days at 82° F (28° C) to develop enough bacteria in your aquarium to keep your ammonia and nitrite levels near 0 ppm. After adding the first fish, ammonia levels will normally peak around two weeks, and nitrite levels will peak around three weeks. During the first month you should keep the fish population very low. The more fish you start with the higher your ammonia and nitrite levels will go. Also keep your feedings light and only once every other day. Your fish will not starve to death, but you could kill them with too much food in an aquarium that does not have an established nitrogen cycle.
It is common for ammonia and nitrite to reach toxic levels in a new aquarium. When this happens, you need to take immediate action. When dealing with toxic levels of ammonia and nitrite, near 100% water changes are in order. Make sure the new water is dechlorinated and the temperature is within a few degrees of the aquarium. Test the aquarium water a couple days after the water change to get a reading on how fast ammonia or nitrite is accumulating. You may need to do additional water changes to keep ammonia or nitrite down, until bacteria populations have reach a high enough level to reduce it faster than it accumulates.

Since nitrifying bacteria live on surface area in the aquarium, some ways to speed up the establishment of the nitrogen cycle is to add the top ¼ inch (.5 cm) layer of substrate, rocks, aquarium decorations, or filter from an established aquarium. Adding a bacteria booster like DrTim's Aquatics “One and Only” will also help establish beneficial nitrifying bacteria to the system.
An aquarium filter cannot oxidize ammonia and nitrite without Nitrosospira, Nitrosomonas and Nitrospira bacteria.
Acidic pH can affect the bacteria's ability to convert ammonia to nitrate. A low pH (below 6.0) can be hostile for a healthy beneficial bacteria population. When cycling an aquarium keep the pH above 7.0. In established aquariums with a pH below 6.0, it is not unusual to see an ammonia reading, due to a decreased bacterial population to process ammonia/ammonium.
If you don't do anything to introduce nitrifying bacteria to a new aquarium, amazingly it will develop on its own, but you will have a greater risk of losing fish.
The best way to introduce beneficial bacteria to a new aquarium is by doing a substrate fauna transplantation. The technique not only introduces nitrifying bacteria, but also anaerobic, as well as many other beneficial species of bacteria that have yet to be studied.
To do a fauna transplantation, you must use some kind of shovel, or deep plastic container to remove a deep scoop of substrate (preferably all the way to the bottom) from an established aquarium and place it gently into a new aquarium, placing new substrate around it. The key is to try not to disturb the substrate layers when removing from the donor aquarium and inserting into the new aquarium. Eventually the bacteria species within the established substrate will colonize the rest of the substrate in the new aquarium.